Autophagy & Neurodegeneration
We aim to define the essential molecular determinants of autophagy in human neurons, and how these determinants play an active role in the pathogenesis of neurodegenerative diseases. To accomplish this, we study physiologic derangements in the autophagy pathway and neuronal health in the context of three disorders hallmarked by dysfunctional autophagy: Parkinson disease, BPAN, and CMT4B3.
Parkinson disease (PD)

Toxic aggregation of the protein alpha-synuclein (aSyn) can be mitigated by degradation through the autophagy pathway. To harness and apply this strategy for translational therapies, we engineer iNeurons derived from biosamples donated by PD patients. We then use our iNeuron platform to investigate the optimal mechanisms for enhancing autophagy in neurons and rescue synucleinopathy.
We are grateful to the National Institute of Neurological Disorders & Stroke (NINDS) and the Levin Family Foundation for supporting this work.
Beta-propellor protein-associated neurodegeneration (BPAN)

Beta-propellor protein-associated neurodegeneration (BPAN) is the most common form of neurodegeneration with brain iron accumulation (NBIA), and is caused by mutations in the autophagy gene, WDR45. It remains unclear why disrupting autophagy through WDR45 predominantly affects neurons, but we are defining cell-specific mechanisms of BPAN pathogenesis and rescue strategies using CRISPR-engineered and patient-derived iNeurons.
We are proud to partner with the Don’t Forget Morgan Foundation in pursuit of this work.
Charcot-Marie-Tooth Type 4B3 (CMT4B3)
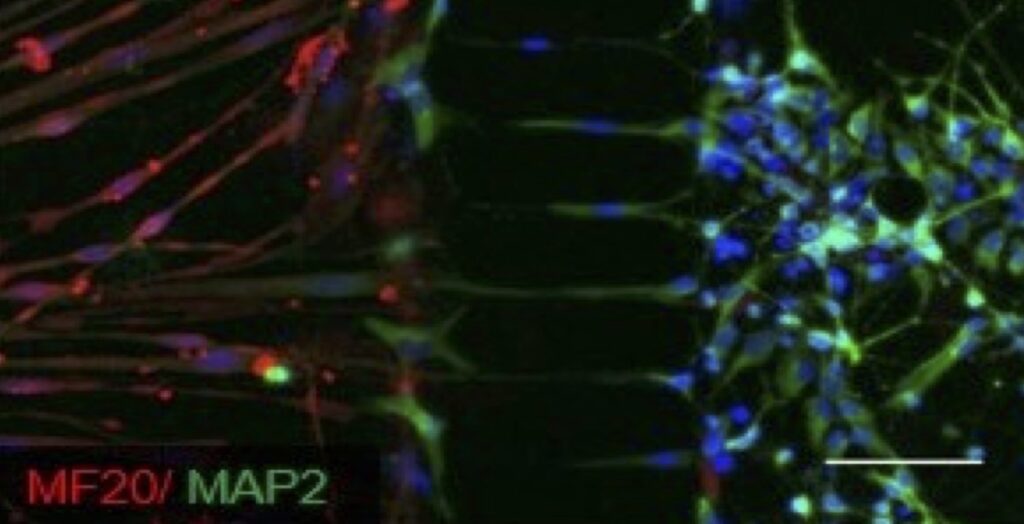
Mutations in the autophagy gene, SBF1, cause the childhood-onset disease, Charcot-Marie-Tooth Type 4B3 (CMT4B3), a type of peripheral nervous system disorder manifesting with distal and progressive weakness and muscle atrophy. SBF1 encodes the pseudophosphatase, MTMR5 (myotubularin-related phosphatase 5), which participates in regulating phosphoinositide metabolism and autophagy induction. We use patient-derived iMotor Neurons, iSensory Neurons, and iMuscle to define the molecular mechanisms connecting SBF1 dysfunction to selective loss of neurons in CMT4B3. We are also testing new precision medicine strategies to target and rescue loss of SBF1 function in patient-derived iMotor Neurons.
We are thrilled to receive the generous support of the CMT4B3 Foundation for this research.

